Menu
About Gateway for Cancer Research
Since its inception in 1991, Gateway for Cancer Research has been harnessing the passion and drive of a global community of brilliant physicians, benevolent philanthropists and brave patients to advance transformational, early-stage clinical trials for all types of cancer. This includes rare cancers for which funding is scarce as well as some of the most prevalent cancers affecting men, women and children today. Gateway exclusively funds Phase I and Phase II research trials, which often mark the first-in-human study of promising new cancer drugs and therapies.
Gateway has invested more than $114 million to advance 234 cancer clinical trials. Conducted at esteemed institutions around the world, these trials have offered HOPE to more than 35,000 patients and families.
Mr. Richard J Stephenson is Chairman of the Gateway Board of Directors, and Dr. Stacie J. Stephenson, serves as Vice Chair.
Gateway funds Phase I and II patient-centered cancer clinical trials that have the potential to shift the paradigm for standard of care. We strive to fund treatment-based studies at the bedside, including all types of cancers.
We advance our mission by funding early phase clinical trials focusing on the following:
New Investigational Therapies
New Indications
De-escalation of Treatment
Mitigation of Treatment Toxicity
Predictive Biomarkers (with a therapeutic component)
Integrative Medicine
In these research areas:
Biomarkers (prognostic or predictive with supportive treatment)
Gene Therapy
Basket Trials
Immunotherapy
Targeted Therapies
We seek to bring urgency to the process of cancer research for patients who are looking for treatments today and are faced with difficult decisions without innovative, life-saving options. Gateway will place increasing emphasis on funding patient-centered and novel research.
At Gateway, it is always and only about the patient. Involvement of patient advocates and patient advocacy groups in the design of the clinical trial, incorporating patient-reported outcomes and quality of life tools, and including patient advocates on research study review committees, are all ways to incorporate the patient’s unique perspective into the research study.
Gateway does not fund the following:
Institutional overhead (indirect costs)
Consultant fees, capital equipment, and computer hardware or software, unless specified in the original application and approved by Gateway
Travel costs, unless approved by Gateway
Basic laboratory research
Pre-clinical animal research
Early prevention or early detection studies
Device testing studies
Retrospective, non-interventional, observational, or certain phases of translational studies
Educational studies
Comparative effectiveness research (CER) studies
Dose-ranging pharmacokinetics that do not include clinical efficacy measures
Correlative studies using human bio specimens may be considered if they impact decision making for patient care.
Pre-Award
Gateway has a variety of core research grant programs designed to facilitate an organization’s broad impact on early phase cancer research, including:
Gateway Traditional Grant Program
Core grants funding early phase clinical trials for cancers of all types at renowned institutions around the world. These grants are typically two (2) to five (5) years in duration with award amounts from $200,000 to $1.5 million.
NCI/ SPORE/ P20 Program
Our collaboration with the National Cancer Institute (NCI)/Specialized Programs of Research Excellence (SPORE)/ P20 Program allows us to fund more patient-centric research.
Gateway collaborates with the NCI seeking proposals from SPORE and P20 awardees that could enable principal investigators and their respective institutions to expand a clinical trial by adding an additional arm, adding new patient cohorts to an existing clinical trial, and/or generating new correlative analysis. Funding cannot duplicate the scope of work of the SPORE award.
This grant program will support translational research through clinical trials conducted by institutions that receive NCI SPORE awards. Gateway will issue grant awards based on an application and review process conducted by Gateway.
Integrative Research
Gateway focuses on funding integrative oncology research that pairs conventional therapies with evidence-based, complementary therapies to manage symptoms and side effects from treatment. This also aims to increase quality of life during treatment.
Society for Integrative Oncology (SIO)
Our collaboration with the Society for Integrative Oncology (SIO) allows us to bring the most promising patient-centered, integrative oncology research proposals forward, Gateway collaborates with SIO to support integrative clinical trials conducted by principal investigators and institutions among its membership. SIO’s mission is to advance evidence-based, comprehensive, integrative healthcare to improve the lives of people affected by cancer.
Decentralized Clinical Trial Initiative (DCTI)
During the COVID-19 pandemic, we launched a new grant program to fund research leveraging site-less, technology-informed trials, with the objective of bringing clinical trials directly to patients’ doorsteps.
Successful DCTI proposals should:
- Advance a treatment-based clinical trial in oncology
- Emphasize the delivery of the investigational treatment in a decentralized setting, ideally home-based
- Define compelling primary and secondary endpoints
- Creatively leverage one or more innovative technologies (telemedicine, wearables, remote monitoring, medical record review, e-consent, etc.)
- Have been reviewed with your CTO Administrative Director for regulatory, data management, and safety considerations related to implementation
- Integrate mobile clinician management, as needed
- Incorporate rigorous and methodical collection, management, and analysis of trial-related data
- Utilize clinical resources in proximity and convenient to patients for any trial-related procedures that cannot practicably be carried out in a decentralized setting (e.g. imaging, interventional radiology, complex infusion, etc.)
- Leverage strategic inter- and/or intra-institutional collaboration, including commercial (e.g. biopharmaceutical, CRO, home health companies, etc.)
An investigator-initiated application from any academic, non-profit, or for-profit organization, institution, or medical center is welcome. An investigator who is ready to conduct a novel, clinical trial in cancer research — one which will have an immediate impact on cancer patients — may apply for funding on behalf of such organization, institution, or medical center. The principal investigator will be required to submit a bio-sketch illustrating their work as a clinician and/or researcher.
The grant award process begins with the submission of a Letter of Intent (LOI) from a research scientist seeking funding for a clinical trial through the secure grants management system portal on Gateway’s website. Gateway’s Research and Grants Committee (R&G Committee) reviews and votes on each LOI based on the scientific rigor of the proposed research and alignment with Gateway’s mission.
If the R&G Committee approves the LOI, the researcher is invited to submit a Full Grant Application (FGA). The FGA is similarly reviewed and scored by the R&G Committee. If the R&G Committee scores the FGA as fundable, it is presented to Gateway’s Board of Directors for final fiduciary and mission-centric review.
The application process is open year-round, but Gateway aggregates and reviews applications three (3) times per year.
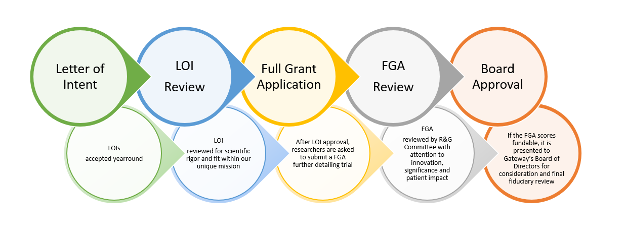
- LOI submission – As part of the LOI, researchers answer basic questions about fit with Gateway’s mission and funding criteria, and provide contact information, high level information related to the proposed research study, a concise summary of the research study rationale, current standard of care, an overview of specific aims/objectives, and proposed research design/approach. LOIs are pre-generated/templated applications and must be submitted before the deadline listed in the portal to be reviewed in Gateway’s upcoming review cycle.
- LOI review – LOIs are assigned to scientists and patient advocates on Gateway’s R&G Committee for review. This review occurs within two (2) to four (4) weeks after the LOI submission deadline.
- Invitation to submit FGA – Applicants who demonstrate that their research study aligns with Gateway’s mission and receive approval from Gateway’s peer reviewers are invited to submit an FGA. Applicants will be given five (5) to six (6) weeks to complete the FGA.
- FGA review – Gateway’s R&G Committee fully reviews each submitted FGA at a convened meeting and gives a score per the NIH scoring model to indicate funding priority. The grants Gateway deems to have satisfactory scores and to be aligned with Gateway’s mission are presented to Gateway’s Board of Directors
- Final approval – Gateway’s Board of Directors reviews the FGAs approved by the R&G Committee and determines whether to give final, fiduciary approval to award a grant (provided that the activation of such grant is contingent upon a fully executed Award Package and completion of all other action items set forth in the Grantee Checklist).
The Research and Grants Committee (R&G Committee) is comprised of medical oncologists, surgical oncologists, clinician scientists, clinical trialists, cancer researchers, and patient advocates from cancer institutions and organizations around the world. The R&G Committee reviews submissions of Letters of Intent and Full Grant Applications, providing scoring (based on the NIH model) and commentary. These are used to create the slate of candidate grantees presented to Gateway’s Board of Directors for final fiduciary and mission-centric approval.
Should an application not score within the fundable window, the commentary is shared with the applicant as feedback, if requested, to assist in the resubmission process.
The R&G Committee also acts as an advocate and driving force for connecting Gateway with like-minded organizations, institutions, and individuals in the field of cancer research.
Please visit our grant management system portal and review the programs offered. If you feel you could qualify, create an account and proceed accordingly based on your area of interest.
Applicants may apply to more than one program if each research study submission is unique (i.e., the same research study cannot be submitted to multiple programs).
You will need to contact Gateway at Research@GatewayCR.org if you need more time to complete and submit your FGA. You must provide an explanation for the requested extension. In its discretion, Gateway may allow a deferment of one (1) grant review cycle, which is approximately four (4) months. Subject to consideration and approval of your request, Gateway will provide you the new deadline for completion of your FGA. A request for deferring longer than one (1) grant review cycle will likely require submission of a new Letter of Intent to be reconsidered by Gateway’s Research and Grants Committee (i.e., starting a new grant application).
The pre-award process, from Letter of Intent deadline to grant approval, is approximately 4 months. Initial IRB/IEC and IND approval is required within 60 days of Gateway’s notification of award. (See “What is included in the Grantee Checklist” below for more details on submission requirements.)
While Gateway does not cap any of its cancer research study grant awards, we typically fund research studies with a budget estimate in the range of $200,000 to $1.5 million. Applicants should apply for the amount they believe is needed to conduct their research study.
Gateway requires supporting documentation and justification for budgets, as well as semi-annual progress and financial reports, throughout the life of the grant. We utilize the NIH salary cap, and the sum of personnel salaries may not exceed 40% of the total grant budget without adequate justification. Gateway has salary limitations for certain personnel. Please reach out to research@gatewaycr.org for more details.
Gateway typically funds research with study periods in the range of two (2) to five (5) years. Applicants should apply for the amount of time they feel is necessary to successfully complete the research study and provide explanation or justification for such time period. While an overall budget and schedule for payment of funds over multiple years will be established, (i) Gateway’s initial funding of a research study is contingent upon a fully-executed Award Package and completion of all other action items on the Grantee Checklist, and (ii) Gateway’s continued funding of a research study beyond the first year is contingent upon satisfaction of established milestones and other factors detailed in the Award Package.
Key Terms & Conditions of an Awarded Grant
The Award Package explains the relationship between, and obligations among, Gateway, the grant award recipient (i.e., the research institution, typically), and the principal investigator for the research study. It consists of:
- Award Letter – provides a high-level explanation of the grant award and each party’s obligations and expectations;
- Financial Snapshot – sets forth the years, milestones, and related dollar amounts contemplated under the award; and
- Terms & Conditions – provides a detailed explanation of the parties’ rights, obligations and expectations under the grant and contemplates what will or should happen if certain future scenarios arise.
The Award Package – once signed and delivered – serves as the contract between Gateway and the grant award recipient, so must be signed by all parties in order to be binding and enforceable. Gateway will not pay any award funds without a fully-signed Award Package in place or without all action items on the Grantee Checklist having been completed.
Gateway has tried to make the Award Package as comprehensive and amenable as possible, given our mission, our advocacy for patients, and our fiduciary responsibilities. We set forth the general concepts of the Award Package as part of the application process. Therefore, by submitting an application, an Applicant is aware of Gateway’s requirements and should not submit an application if such concepts are not acceptable by the Applicant or his/her institution.
We prefer that changes not be made to the Award Package. As this is a contract between Gateway and the award recipient, we are open to considering certain requested changes from an award recipient. However, Gateway considers some concepts in the Award Package to be “non-negotiable.” An award recipient’s refusal to agree to such terms, as we have proposed them, could be a reason for Gateway to rescind a grant award. Such items, each more fully described in separate FAQs, include (but are not limited to) the provisions in the Terms and Conditions regarding: compassionate use, patient voices, royalties related to commercial exploitation of inventions, practical application, and submission of periodic reports.
For specific questions about aspects of the Award Package, or possible changes thereto, please email us at Research@GatewayCR.org.
Every patient who participates in a clinical trial plays a critical role in conquering cancer on behalf of all of us. At Gateway, we feel a deep responsibility to every one of these patients to create a shared sense of urgency in the field of cancer treatment, and to improve the overall standard of care by driving transformative discoveries. Therefore, Gateway expects patients to remain on the investigational drug as long as a doctor indicates clinical benefit. Gateway recognizes that this is regulated by the U.S. Food and Drug Administration (FDA). The FDA stipulates that investigational drugs should be continued when it is clear that patients may continue to benefit from the treatment, the therapy can be given safely outside of the clinical trial setting, no other alternative therapy is available, and the sponsor agrees to provide access to the drug. Applicants for a Gateway grant are expected to consider Gateway’s viewpoint in the design and conduct of their clinical trials.
Gateway recognizes that each patient’s approach to sharing (or not) such patient’s story is different. Gateway never requires that patients share their stories or identify themselves, but we know from experience that many patients want to share and/or connect with those who helped to fund their particular clinical trial. And, the patients willing to share their experiences with Gateway enable Gateway to raise more money to help more patients.
Patients may elect to share their stories in a variety of ways (e.g., email, letter, interview, recorded video) and can provide as much, or as little, detail as they desire. Patients may reach out to Gateway directly, or might contact the institution or principal investigator to inquire about sharing. We also appreciate anecdotes or snippets of patients’ experiences shared with us by the principal investigator, either directly with the Gateway team or via periodic reports, that do not specifically identify the patient.
As part of the Award Package, we simply request that a reasonable effort be made by the award recipient to make patients aware of the opportunity to share their stories with Gateway. Gateway will accommodate requests to keep the patient unidentified and/or share only aspects for which the patient specifically consents.
Our Terms and Conditions contemplate that, as between Gateway and the grantee institution, the institution (i.e., the award recipient) will typically own any and all inventions, protocols, innovations, discoveries, and other work product, technologies and intellectual property created in connection with the research study. The complete definition of these “Inventions” is set forth in our Terms and Conditions, which will be provided as part of the Award Package.
A primary objective of Gateway in awarding grants is to facilitate the creation, utilization, and commercialization of Inventions in the interest of Inventions being made widely available to the public as expeditiously as possible for the purpose of diagnosing, treating and/or curing cancer or otherwise facilitating the care or comfort of cancer patients, which purpose we call “Practical Application.” Accordingly, we require our award recipients to provide us notice of Inventions that may have a Practical Application, and of any patent applications and other applications for registration of such Inventions. We also require each award recipient to use diligent efforts to commercialize such Inventions in a timely manner to achieve Practical Application.
We require award recipients to pay a royalty to Gateway equal to an agreed upon percentage of all revenues and other consideration in connection with all transactions with third parties with respect to Inventions and/or products or services based on Inventions, deducting only reasonable, out of pocket costs incurred directly by the award recipients in connection with negotiation of such transactions. We allocate all royalties collected back to funding Gateway-funded research studies.
If, within two (2) years of notifying Gateway of an Invention having the potential to achieve Practical Application, either no effective steps to achieve Practical Application have been taken or any such efforts have been discontinued, then the award recipient must make any reasonable disposition of the Invention as Gateway may direct. This is to help ensure any new innovative treatment options are brought to every patient with urgency.
Yes. In fact, also in the interest of swiftly disseminating information regarding potentially useful information to the cancer patient and care communities, our Terms and Conditions require you to use good faith efforts to publish, in scientific or academic journals, all results and findings of your research study serving the aims or purposes of your study plan. If no such articles have been published within one (1) year following the end of the term of your grant, Gateway may publish the relevant results and findings.
Gateway’s principal office is located in the Chicago, Illinois metropolitan area. If the award recipient refuses to agree to Illinois as governing law in the Award Package, then Gateway may, in its discretion, agree to remove all references to a particular state (i.e., “go silent”) but will not agree to designating a state other than Illinois. If the award recipient is located outside the United States, Gateway will require that the Award Package be governed by Illinois law.
Post-Award
Once approved, Gateway will provide you with an Award Package, which will include an Award Letter, Financial Snapshot, and Terms & Conditions. Gateway will work with you and/or your institution to execute the Award Package. You will then use Gateway’s Grant Management System to submit all required documents to fully activate the grant. The Grant Management System is also used for all post-award activity, including semi-annual reporting and financial grant tracking.
The following items comprise the Grantee Checklist:
- Verification of principal investigator’s contact information and address
- Institution’s check/payment information
- Institution’s public relations contact information
- Institution’s foundation relations contact information
- Updated research study budget (if applicable)
- Updated research study timeline (if applicable)
- Clinicaltrials.gov registration identifier
- Fully executed Award Package (e.g., Award Letter, Financial Snapshot, Terms & Conditions)
- Clinical trial agreement (if applicable)
- Institution’s IRB/IEC initial, (latest) amendment, or continuing review approval letter
- IND final approval including FDA “Study May Proceed”, “Remove Clinical Hold”, or “Acknowledge/Exempt IND” letter (if applicable)
- Principal investigator’s headshot
- IRB/IEC approved Informed consent/assent form
- Wiring instructions
- Institution’s W9
- Seed funding invoice
The Grantee Checklist must be successfully completed within 60 days of Gateway notifying you of an award. If the Grantee Checklist is not successfully completed during this timeframe, Gateway has the right to rescind the award, so that Gateway may allocate funds to other studies that are waiting. It is recommended that you start on the Grantee Checklist immediately after being notified of your award, as certain aspects may require input and approval from your institution, which we have seen cause delays.
Gateway decides the total amount potentially payable by Gateway for the particular grant, which is considered the “Total Grant.” The Total Grant is split into “buckets” based on the agreed upon length of the grant, expected number of patients, and anticipated milestones, as outlined in the Award Package.
Gateway uses a patient accrual methodology, which is factored into calculations of the buckets and is based upon the approved number of patients expected to be enrolled and treated in the research study, as more specifically outlined in the Award Package. Patient impact must be demonstrated through semi-annual and close-out reports to Gateway, which, if complete and satisfactory to Gateway, may, in Gateway’s discretion, trigger payment of the next bucket of funds.
To facilitate start-up of the applicable research study, Gateway provides 20% of the Total Grant as “seed money”. Upon the potential award recipient’s successful completion of the Grantee Checklist, Gateway may activate the grant and pay such seed money.
The payment of each funding bucket is contingent upon approval by Gateway’s Board of Directors, which will rely on the award recipient demonstrating patient impact and treatment through reporting. Gateway’s payment methodology is not negotiable.
Patient Accrual Milestone Amount = (Total Grant – (20% Seed Money + Site Visit Expenses + Closeout Report Payment)) / Total Number of Patients Expected
Example:
| Grant Term (Years) | 5 |
| Total Grant | $1,000,000.00 |
| Total Site Visit Expenses (over Grant Term) | ($5,000.00) |
| Seed Money | ($200,000.00) |
| Closeout Report Payment | ($20,000.00) |
| Net | $775,000.00 |
| Total Number of Patients Expected | 100 |
| Patient Accrual Milestone Amount | $7,750.00 |
No, patients must be treated under the research study to be considered in the patient accrual calculation and be documented in the required patient log that is part of the semi-annual reporting requirements.
Screen-fails, patients that drop prior to treatment, or patients that only complete screening visits are ineligible under the patient accrual methodology. Gateway will consider patients who receive treatment and are subsequently removed for various reasons (i.e., SAE, DLT, patient electively withdraws from the research study, rapid disease progression, etc.) under the patient accrual methodology.
If the total number of patients expected to be treated to power the research study changes during the course of the research study, please email us at Research@GatewayCR.org and we will consider your request. Changes will not be made, and should not be requested of Gateway, after completion of patient accrual.
You will need to contact Gateway at Research@GatewayCR.org to request a change to, or replacement of, the PI for the research study. You must provide a biosketch of the proposed new PI, justification for the requested change (which should be provided on the grantee institution’s letterhead), and IRB/IEC approval of the proposed PI change. The request should be made to Gateway within 30 days of when the change is anticipated to take effect. Gateway is not required to accept a change to the PI, but Gateway will review and consider such requests. If Gateway approves of changing the PI, then an amendment to the Award Package effecting such change will be prepared to be signed by all parties.
You will need to contact Gateway at Research@GatewayCR.org if your research study is taking longer to complete than originally set forth in your grant application. Please submit your request for a timeline extension on the grantee institution’s letterhead, with a detailed explanation for the request, at least 30 days before the research study end date listed in your grant application. Gateway is not required to grant a timeline extension, but Gateway will review and consider such requests. If Gateway approves of extending the timeline, then an amendment to the Award Package reflecting such extension will be prepared to be signed by all parties.
SARs are due twice each year — on January 31 and July 31. They can be completed in the same Grant Management System that was used to apply for your grant.
New Grantees (first SAR)
- July 31 – for the reporting period Grant Activation through June 30
- January 31 – for the reporting period Grant Activation through December 31
Existing Grantees (subsequent SARs)
- July 31 – for the reporting period January 1 through June 30
- January 31 – for the reporting period July 1 through December 31
- Administrative information
- Patient accrual summary
- De-identified patient log (See separate FAQ below for more details.)
- Study milestone and timeline progress
- Recruitment efforts
- New insights to share over the past six months
- Principal investigator video update (2-5 mins)
- SAR payment request/invoice
- Trial expenditures to date from Gateway funded budget
The following (if applicable):
- Updates to key study personnel
- Any modifications to the approved/funded protocol
- Updates about the activation of sub-sites
- Any publication updates
- Reporting of SAEs or DLTs related to treatment (after they have been addressed and reconciled)
- Updates to IRB/IEC approval (continuing review)
- De-identified patient quotes, comments, or anecdotes
Gateway provides the template you will use to submit the de-identified patient log. The information to be included is:
- A patient study ID/# (this cannot be a MRN or any other protected health information)
- Institution patient was seen at (if multi-center)
- Cancer/tumor type
- Patient’s status on the clinical trial (i.e. screening, screen failure, in treatment, etc.)
- Initial reporting period date of the patient
- ICF completion date
- C1D1/treatment Day 1 date
- Completion date/off-study date
- Best response (if applicable)
- Any additional comments you would like to provide (do not provide protected health information)
Gateway does not request, and does not want to receive, any information that would be considered “protected health information” under the Health Insurance Portability and Accountability Act of 1996 (HIPAA). The information provided to Gateway in the patient logs should be de-identified pursuant to HIPAA.
Generally, the information contained in the semi-annual reports is for Gateway’s internal review and review by its Board of Directors. Information is not shared externally, unless: (i) the Grantee Institution specifically consents to such sharing; (ii) the award is being co-funded by another organization in collaboration with Gateway (for which the Grantee Institution would have previously been made aware); or (iii) the question posed in the report indicates information is intended for external use, and the principal investigator provided information for such purpose.
Help fund one patient for one day in a Gateway-funded clinical trial
Fund NowFund One Day
It costs just $19.62 per patient per day in a Gateway-funded cancer clinical trial.
Help us fund another day of research, another week or more. You can even mark your gift with a special date, such as an anniversary, birthday, wedding, bar/bat mitzvah or graduation.
